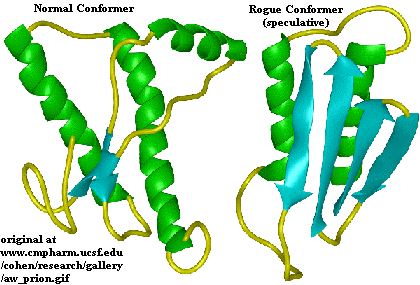
 The normal protein shows the prevalence of
The normal protein shows the prevalence of  helices, in green, and the rogue protein (PrPSc) shows increase in
helices, in green, and the rogue protein (PrPSc) shows increase in 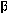 pleating, in blue.
pleating, in blue. So, what are these curious particles? Essentially, they are nothing more than a bent protein. The name "prion" is really shorthand for the words "proteinaceous infectious particles."(Prusiner Prion 48) These proteins have a specific amino acid composition, although their shape can differ. It is these differences in shape that lead to the problem.
So, what are these curious particles? Essentially, they are nothing more than a bent protein. The name "prion" is really shorthand for the words "proteinaceous infectious particles."(Prusiner Prion 48) These proteins have a specific amino acid composition, although their shape can differ. It is these differences in shape that lead to the problem. helices, because they can be manipulated by enzymes and chemicals. The second form is much more rigid and stable.(Cohen et al 531) Instead of the helix, it forms a pleated sheet, called a
helices, because they can be manipulated by enzymes and chemicals. The second form is much more rigid and stable.(Cohen et al 531) Instead of the helix, it forms a pleated sheet, called a 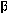 sheet, which isn't easily destroyed by the body. Prions come in these two forms. The normal protein, PrPC, is composed largely of
sheet, which isn't easily destroyed by the body. Prions come in these two forms. The normal protein, PrPC, is composed largely of  helices so that the body can create and destroy them without difficulty. The rogue protein, or disease causing prion, forms
helices so that the body can create and destroy them without difficulty. The rogue protein, or disease causing prion, forms 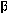 sheets instead.(Prusiner Prion and BSE 245)
sheets instead.(Prusiner Prion and BSE 245)
 The normal protein shows the prevalence of The normal protein shows the prevalence of  helices, in green, and the rogue protein (PrPSc) shows increase in helices, in green, and the rogue protein (PrPSc) shows increase in 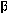 pleating, in blue. pleating, in blue. |

